
In Chemical Process Industries (CPI), we use Agitated Batch Reactors for various product manufacturing in fine chemicals, specialty chemicals, APIs, etc. These types of reactors are easy to use and provides flexibility in operation in comparison with continuous reactors. Moreover, we can produce variety of products as per the market demand. However, operating cost for batch operation is more than a continuous operation. For instance, we can use these batch reactors for liquid-liquid, liquid-gas, liquid-liquid-solid and liquid-gas-solid phase reactions. In this article we will take an example, to understand the material & energy balance for an agitated batch reactor process.

Table of Contents
Let us assume a homogeneous liquid phase non-catalytic reaction. In this reaction two organic raw materials, chemical ‘A’ and chemical ‘B’ reacts to form chemical ‘C’. This is an exothermic reaction and raw material ‘A’ is limiting reactant. Chemical ‘B’ consumption is 1.25 times of reactant ‘A’. Heat of reaction is 150 kcal/kg of reacted ‘A’.
In this process equilibrium conversion of the reaction is 85% on the mass basis for reactant ‘A’. This reaction takes place at 85 0 C and atmospheric conditions. Selectivity of the reaction is 95% on mass basis. And remaining 5% of reacted ‘A’ converts into high boiling tar like material. This residue composition is as below which is sent for incineration. The calorific value for residue is 7500 kcal/kg approximately.
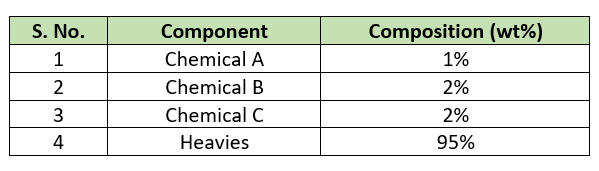
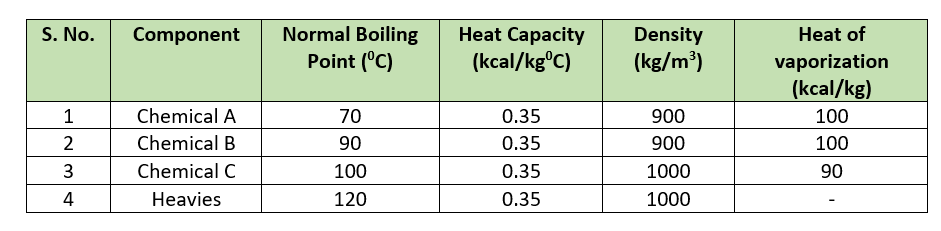
For our batch reactor process calculations, we need physical and chemical properties for the chemicals are in below table.
A (liq.) + B (liq.) —-> C (liq.) at 85 0 C and atmospheric pressure, below is the process flow diagram for our batch reactor system.
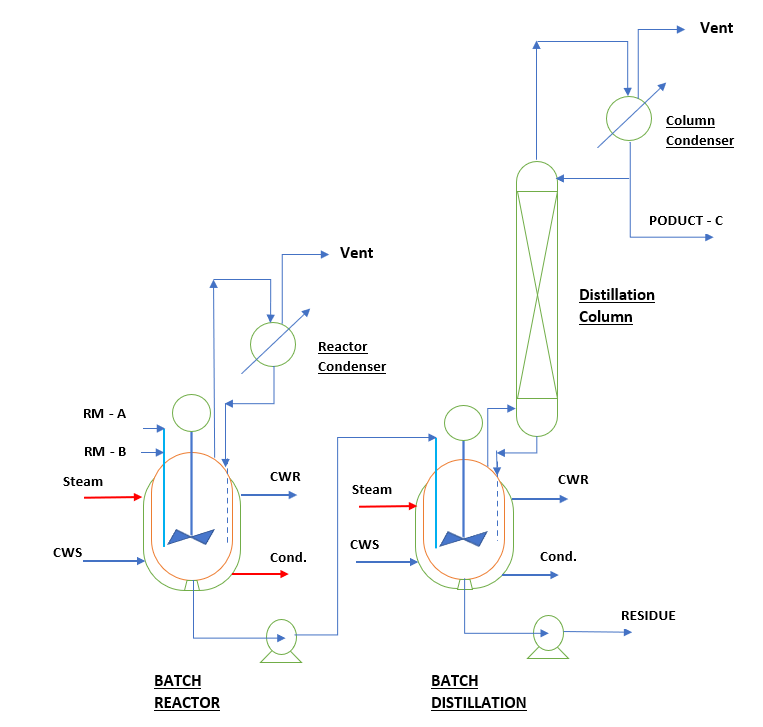
Here,
RM – Raw Material
CWS – Cooling Water Supply
CWR – Cooling Water Return
Cond. – Steam Condensate
This process includes two steps first is reaction and second is batch distillation. The material balance for per batch will be as below.
Heating utility for our process is 3.5 bar steam at saturated conditions. The temperature of the steam is 139 0 C and latent heat is 513.5 kcal/kg.
Therefore, total steam requirement for total batch processing will be Q = Q1 + Q2 + Q3 + Q4 + Q5 + Q6 = 76.7+315.5+723.0+6303.8+225.3+351.3 = 7995.6 kg/batch. Considering 5% steam loss actual steam requirement will be Q’ = 1.05*Q = 8395 kg/batch.
Cooling water flow rate requirement will be based on when our reaction is going on and pure product draw is going on. As this will be maximum requirement any point of time during the process.
Total flow rate for cooling water pump will be W = w1 + w2 + w3= 937.5 + 40466 + 5000 = 46403.5 kg/h or 46.4 m3/h.
Cooling water pump will be 50 m3/h and 30 m head. Hence power consumption will be P1 = m*9.81*h/(3600*pump efficiency) = 50000*9.81*30/(3600*0.75) = 5450 W = 5.45 kW. Total consumption per batch will be P1’ = P1*Batch Cycle Time = 5.45*27 = 147.15 kW/batch.
Reactor and distillation vessel agitator motor power is 5.5 kW. Hence power consumed by agitator motors will be P2 = Reactor agitator* operating hours + Distillation agitator* operating hours = 5.5*570/60 + 5.5*1050/60 = 148.5 kW/batch.
Other transfer pumps and auxiliary power requirement P3 = 60 kW.
Total power requirement will be P = P1’ + P2 + P3 = 147.15 + 148.5 + 60 = 355.65 kW.
RM – A consumption norm = Consumption A/Production of C = 1730/3607.2 = 0.4796 kg/kg
RM – B consumption norm = Consumption B/Production of C = 2162.5/3607.2 = 0.5995 kg/kg
Residue generation norm = Residue generation/Production of C = 191.3/3607.2 = 0.053 kg/kg
Steam consumption norms = Q’/3607.2 = 8395/3607.2 = 2.327 kg/kg
Power requirement will be = P/3607.2 = 355.65/3607.2 = 0.099 kW/kg
Listing down unit operation wise all the steps in batch reactor process system and adding the time taken in each process step provides the cycle time for that process step. Below is the table and sample working for your understanding:
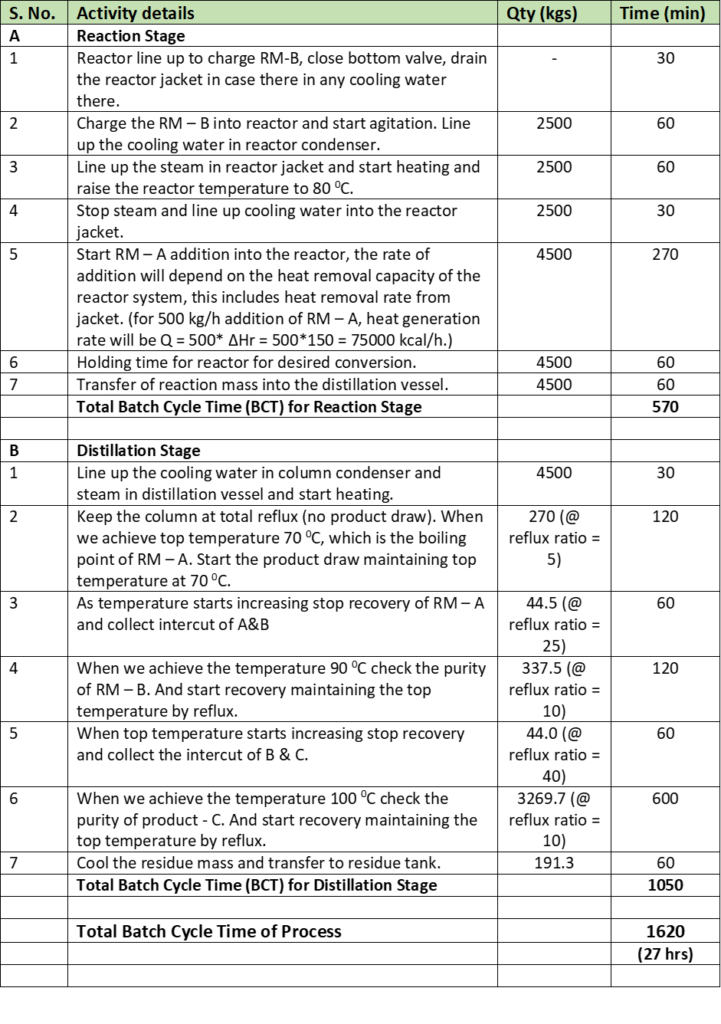
In our example there are two process steps, first is reaction and second is distillation. From above table we can see in batch reactor total time cycle is 570 min and in distillation step batch cycle time is 1050 min. Therefore, effective batch cycle time for this process will be whichever is highest and here it is distillation step. So, batch cycle time or BTC of this batch process is 1050 min. While overall batch time cycle is (570+1050= 1620 min).
You can use this procedure to carry out the material and energy balance for the batch reactor plant. For your work you will get a technology package from your R&D department. In this you will get all the information regarding reaction and downstream requirement. After this we do the equipment design for equipment. In our example various equipment are such as reactor, reactor condenser, transfer pump, distillation vessel, distillation column, column condenser, etc.
Next, we design control system requirement for reactor temperature, distillation column temperature, steam flow and reflux flow. Pipe line sizing for process, cooling water, steam and condensate.
P&ID development, equipment layout and elevation drawings are developed subsequently. In my future post we will go through all the steps.